Neutrophils are the most abundant leukocytes and essential components of the immune microenvironment. Myeloproliferative neoplasms (MPNs) are characterized by excessive myelopoiesis and serious complications, such as thrombosis, myelofibrosis and leukemic transformation. Neutrophils and hematopoietic stem cells (HSCs) carrying the most common MPN driver mutation (JAK2 V617F) are protected from apoptosis. Moreover, mutant neutrophils aggregate with platelets and interact with activated endothelium, increasing thrombosis risk in MPN (Blood 107:3676; Thromb Res 129:263; Sci Transl Med 10:eaan8292; Leukemia 33:2544). However, possible pathogenic interactions between heterogeneous bone marrow (BM) niches in MPN (Nat Cancer doi:10.1038/s43018-023-00607-x) and neutrophils are understudied.
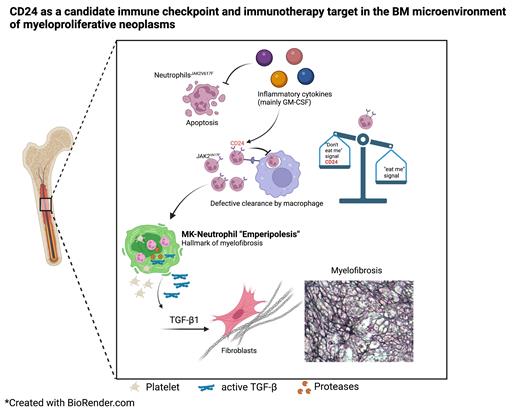
Circulating HSCs and leukocytes home daily to the BM (Nature 452:442), where apoptotic/senescent neutrophils are cleared by macrophages (Immunology 125:281; Cell 153:1025). We found that senescent neutrophils carrying JAK2 V617F or CALR del52 mutation evade normal clearance and accumulate in the BM of MPN mice. The homeostatic recognition and efferocytosis of neutrophils by macrophages rely on the balance of “eat-me” and “don't-eat-me” signals. Supervised analyses of two independent human JAK2 V617F+ MPN granulocyte RNAseq datasets (Blood 123:e123; Blood 134:199) showed specific upregulation of CD24 - reportedly a don't-eat-me signal in solid tumors (Nature 572:392). CD24 expression was high in granulocytes from MPN transformed into AML and in the JAK inhibitors ruxolitinib and fedratinib-resistant HEL cells (Nat Cancer 4:108). Unlike CD274 or CD47, which are extensively studied in myeloid malignancies, our data showed that CD24 expression was high in JAK2 V617F+ human or mouse neutrophils. Functional blockade in co-culture with macrophages or after in vivo adoptive transfer showed that CD24 serves as a don't-eat-me signal in neutrophils.
Given that oncogenic driver mutations lead to the constitutive activation of cytokine-regulated JAK-STAT signaling, we investigated the effect of cytokines activating this pathway. We found that high GM-CSF in MPN BM induces CD24 expression and prevents neutrophil clearance via JAK-STAT5 signaling. Consequently, ruxolitinib normalized GM-CSF-induced CD24 expression and decreased human and mouse neutrophils in MPN.
Our study showed that accumulation of mutant senescent neutrophils in the BM triggered abnormal interactions within the BM microenvironment. Mutated neutrophils were found closer to Megakaryocytes (Mk) and ~30% of Mks showed neutrophils transiently invading their demarcation membrane system through a process termed “emperipolesis”, which increases thrombopoiesis and intercellular exchange between neutrophils and Mks (Elife 8:e44031) and frequently occurs in diseases associated with myelofibrosis, such as MPN (Blood 104:3573) and grey platelet syndrome (Blood 124:3624). To examine whether mutated neutrophils affect platelet production, we transferred wild-type or JAK2 V617F+ senescent neutrophils and found that JAK2 V617F+ mutated (but not WT) senescent neutrophils increased Mk emperipolesis and circulating platelets.
Supervised RNA-seq analysis of CD24 hi JAK2 V617F+ human neutrophils highlighted their pro-inflammatory signature. Furthermore, CD24 expression positively correlated with neutrophil degranulation signatures and proteolytic enzymes that activate latent pro-fibrotic TGF-β in Mk, subsequently triggering myelofibrosis. Taken together, these findings suggest that accumulation of senescent neutrophils might lead to their pathogenic interactions with Mks or platelets, which may increase thrombocytosis and myelofibrosis risk in MPN. To test this hypothesis and the therapeutic potential of CD24 inhibition, MPN mice were chronically treated with CD24 blocking antibody. This treatment improved thrombocytosis, decreased platelet-neutrophil complexes, restored normal clearance of mutated senescent neutrophils and reduced emperipolesis levels which, in turn, decreased active TGF-β, ultimately improving myelofibrosis.
This study reveals defective neutrophil clearance as a cause of pathogenic microenvironmental interactions that may increase thrombosis and myelofibrosis risk, and postulate CD24 as a candidate target for innate immune checkpoint in myeloid malignancies.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal